Chemistry 346 Hope College
ABSORPTION SPECTRA OF CONJUGATED POLYENES
Abstract
Ultraviolet-visible spectroscopy is used explore the electronic structure of several conjugated polyenes, and a Particle in a Box model is used to extract structural information.
Background Reading
· D.A. McQuarrie, Quantum Chemistry, §3-5, "The Energy of a Particle in a Box is Quantized", pp. 85-88.
Background
Conjugated polyenes are characterized by a structure with alternating p-electron character (double bonds). It is known that electrons are delocalized over the entire conjugated system. By changing the size of the conjugated system, control can be exerted over the “box” in which the electrons may move. This aspect of conjugated molecules suggests that the “Particle in a Box” quantum mechanical model may reasonably describe their electronic energy levels, measured through ultraviolet/visible spectra. In all spectroscopy experiments, one is primarily concerned with the energy level transitions that will occur with the introduction of light. In the case of the polyene spectra, the observed transition will be between the two lowest electronic states (denoted as the S1 ¬ S0 transition).
Polyenes are perhaps the most ubiquitous pigment in nature, occurring in organisms ranging in complexity from bacteria to humans. Their primary role is as protective agents, helping to prevent the oxidation of key chemical species involved in events such as respiration. This class of anti-oxidants are referred to as carotenoids. As the name suggests, b-carotene is a key carotenoid in plants. Carotenoids in photosynthetic systems also help to absorb sunlight, passing the energy on to chlorophylls, which begin the conversion of this electronic energy into chemical energy (photosynthesis). Polyenes also make up the photoreceptors in many organisms, including the rods and cones of the human eye. Clearly, it is crucially important to understand the photophysics of polyenes. The complex electronic structures of naturally occurring polyenes such as carotenoids are currently the focus of intense study in many laboratories. We will focus on the simpler electronic structures of synthetic polyenes, which are reasonably described by the Particle in a Box model.
In order to understand how the Particle in a Box model is applied to conjugated systems, the application of the analysis to the hexatriene molecule is detailed below. For hexatriene, there are three pi bonds and thus there are six pi electrons (N=6). Two electrons are placed in each energy level (Pauli Exclusion Principle) starting from the lowest energy level (Aufbau Principle).
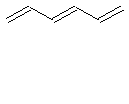
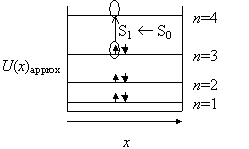
For hexatriene, it can be seen that the S1 ¬ S0 transition corresponds to a n=4 ¬ n=3 transition in the Particle in a Box model. The wavefunctions and energy for the Particle in a Box model are
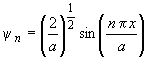 (1)
(1)
and
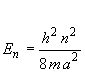 (2)
(2)
where n = 1, 2, 3 ...
The box length a can be calculated from the energy difference for the S1 ¬ S0 transition.
![]() (3)
(3)
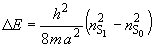 (4)
(4)
where nS0 = N/2 and nS1 = N/2 + 1. Therefore, DE can be expressed in terms of N, the number of pi electrons
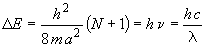 (5)
(5)
which may be solved for the box length
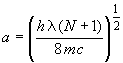 (6)
(6)
The box length, a, may be also be related to the polyene structure with the intuitive formula
a = (N · l) + E (7)
where N is the number of pi electrons (which equals the number of C atoms in the polyene chain), l is the C–C bond length in the chain, and E is the effective size of the endgroups. (See Appendix I, p. 20.)
In this experiment, you will use the wavelength of the S1 ¬ S0 transition with Eq. 6 to determine box length for each polyene consistent with the Particle in a Box model. These box lengths for a family of structurally similar polyenes (with different N) will then be used to estimate l and E for that family.
Procedure
Sign up for two hours of time on the Cary-5E UV-Vis Spectrophotometer in the Spectroscopy Lab (Peale 233).
Turn on the computer first and run the Scan program. Sign in logbook. After the Scan program has loaded, verify that the sample compartment is empty and turn on the Cary spectrophotometer. Wait for the Cary to initialize itself. (If errors occur, click OK and allow the Cary to try again. The instrument is initialized when the Abs and Wavelength displays in the upper corners of the screen are active.) Report any instrument error messages in logbook. Set the following in the Setup dialog box:
Cary tab: |
Cary tab (con’t): |
The samples are pre-mixed and located in the spectroscopy laboratory hood. Handle the quartz cuvettes with care; they are fragile. Touch only the frosted sides, taking care to not get fingerprints on the windows. The windows may be cleaned by gently wiping with a Kimwipe moistened with methanol. Take no more than one cuvette volume of stock solution for each compound. Be careful not to contaminate the stock solutions; use the separate dropper provided for each solution. Seal each bottle immediately after use.
Insert the sample in the front cuvette holder and the solvent reference in the rear holder. Click the Start button to initiate a scan. For the Save As location, navigate to the C:\Chem346 directory and use your initials and the dye code as the filename, e.g., BPK-IA. For the Sample Name, use the dye name and code, e.g., 1,1’-diethyl-2,2’cyanine iodide (I-A). Record UV-VIS spectra for each of the polyenes that have been assigned to your group. Dispose of used dye solution in the organic waste disposal container provided in the hood.
Print the full UV-VIS absorption spectra of each of the polyenes. Also print an extra copy of the full spectra for your notebook. Expand the horizontal and vertical scales about the S1 ¬ S0 peak in each spectrum of the polyenes, and print the expanded spectrum of each compound so that the wavelength of the band origin can be determined. (Graph: Axes Scales is useful for adjusting the horizontal and vertical axis scales.) Print a graph that has all of your polyene spectra superimposed. (Graph: Trace Preferences is useful for overlaying spectra.)
When finished, turn off the Cary and computer. Rinse the cuvettes out several times with methanol. Let them dry before putting the cuvettes away in their container. Clean up any mess that might have been made. Leave the area very clean.
Lab Report
Follow the format given with the course syllabus. The following guide should help.
Introduction. State the purpose of this laboratory experiment. Indicate what quantities are to be determined, and how this will be done. Briefly describe what quantum mechanical model will be used to interpret the absorption spectra. Give the equations that will be used to treat the data, clearly indicating what each variable represents.
Experimental. Briefly summarize the procedure followed and include all details relevant to the taking of the spectra. Include some discussion on the internal workings of the spectrophotometer. What are the advantages and disadvantages of this type of spectrophotometer?
Spectra. Include the expanded spectrum for each polyene. The expanded spectrum should show the band origin extrapolation. Include the superimposed polyene spectrum, with each polyene clearly labeled. Each spectrum should have a figure number, descriptive caption, and labeled axes.
Results. Determine the wavelength of the band origin l for the S1 ¬ S0 transition in each of the polyenes. Post your results (dye code, compound name, and band origin) for each polyene measured on ChemBoard for use by the entire class.
Comment on each recorded spectrum. Calculate the effective box length for each polyene in the class-collected data set using Eq. 6. Prepare a table for the class-collected data set with columns for compound code, compound name, total number of p‑electrons in the straight chain conjugated part of the molecule N, band origin l, and effective box length a. Show one sample calculation for a polyene whose spectrum you recorded.
For each polyene family, construct a plot of box length against number of C atoms in the polyene chain, fit this data with a straight line (Eq. 7), and extract experimental determinations of the C–C bond length and the size of the endgroups. Perform a similar analysis and construct a plot using the entire polyene data set. Report your results in a table.
Standard Deviation. Determine and report the standard deviation of the experimentally determined C–C bond length l and endgroup size E for each family as well as the entire data set. Report your final values and standard deviations of the C–C bond length and endgroup size in a table.
Discussion. Use your results to evaluate the usefulness and accuracy of the Particle in a Box model for polyene electronic transitions. Qualitatively evaluate the model by discussing the observed correlation between polyene chain length and S1 ¬ S0 transition wavelength. Quantitatively evaluate the model by comparing your experimental C–C bond lengths to literature values for various types of C–C bonds (single, double, conjugated, etc.). Discuss the validity of the experimentally determined endgroup sizes. Compare the differences between experimental and literature C‑C bond lengths to your experimental uncertainties. Compare the uncertainty obtained from the individual polyene analyses with that obtained for the entire data set. What limits the quantitative accuracy of the analysis? Conclude your discussion with an assessment of at least two specific strengths and two specific weaknesses of the Particle in a Box model for electronic transitions.
References. Cite all literature references, including page numbers.
Notebook. Attach photocopies of all pages in your laboratory notebook that are relevant to the experiment.
Appendix I:
Structures and Names of Polyenes Under Study
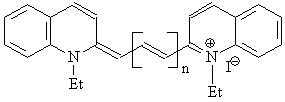
n = 0: 1,1’-diethyl-2,2’-cyanine iodide (I-A)
n = 1: 1,1’-diethyl-2,2’-carbocyanine iodide (I-B)
n = 2: 1,1’-diethyl-2,2’-dicarbocyanine iodide (I-C)
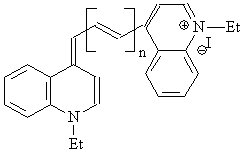
n = 0: 1,1’-diethyl-4,4’-cyanine iodide (II-A)
n = 1: 1,1’-diethyl-4,4’-carbocyanine iodide (II-B)
n = 2: 1,1’-diethyl-4,4’-dicarbocyanine iodide (II-C)
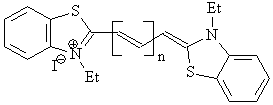
n = 0: 3,3’-diethylthiacyanine iodide (III-A)
n = 1: 3,3’-diethylthiacarbocyanine iodide (III-B)
n = 2: 3,3’-diethylthiadicarbocyanine iodide (III-C)
n = 3: 3,3’-diethylthiatricarbocyanine iodide (III-D)
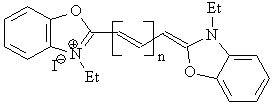
n = 1: 3,3’-diethyloxocarbocyanine iodide (IV-B)
n = 2: 3,3’-diethyloxodicarbocyanine iodide (IV-C)
n = 3: 3,3’-diethyloxotricarbocyanine iodide (IV-D)
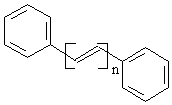
n = 1: trans-1,2-diphenylethene (V-A)
n = 2: trans-1,4-diphenyl-1,3-butadiene (V-B)
n = 3: trans-1,6-diphenyl-1,3,5-hexatriene (V-C)
n = 4: trans-1,8-diphenyl-1,3,5,7-octatetraene (V-D)