The heat of vaporization of a liquid is a useful thermodynamic quantity because it allows the calculation of the vapor pressure of the liquid at any temperature.
Two practical applications of heats of vaporization are distillations and vapor pressure:

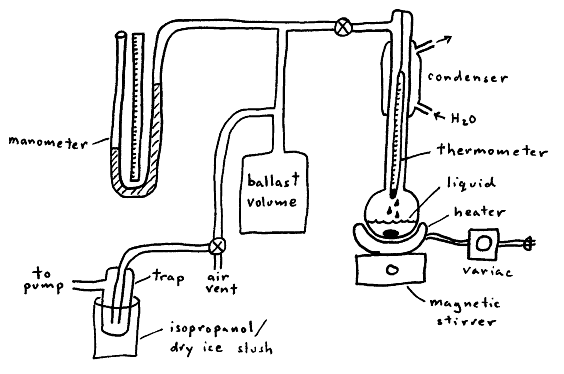
In this apparatus, the pump and air vent allow control the system pressure. The liquid is heated until it boils under the applied pressure of the system. Since a liquid boils when its vapor pressure equals the applied external pressure, the vapor pressure of the liquid at a given temperature is known by reading the manometer and thermometer. (The only unusual aspect of this experimental arrangement is that pressure is the independent variable, while temperature is the dependent variable.) The purpose of the condenser to reduce the amount of organic vapor entering the rest of the system, and the purpose of the ballast volume is to absorb small volume changes as the system is heated or cooled.
Two phases in equilibrium at constant pressure and temperature have the same Gibbís free energy. Recalling that dG = Vdp - SdT yields
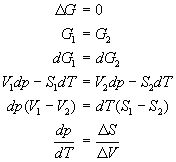
For a phase transition occurring at constant temperature and pressure, the definition of entropy dS = dq/T implies that D Stransition = D Htransition/T. Thus,
|
|
The Clapeyron equation is valid along any phase co-existence line. It explains, for example, that the solid-liquid phase co-existence line for water has a negative slope in a p vs. T phase diagram, since the change in volume in going from ice to water is negative (ice floats in water).
When the Clapeyron equation is applied to liquid-vapor phase co-existence, several simplifying assumptions can be made.
First, since the volume of a gas is much greater than the volume of a condensed phase,
![]()
Second, the gas is assumed to obey the ideal gas law
![]()
which allows the Clapyron equation to be simplified as follows
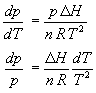
Third, DH is assumed to be independent of temperature and pressure, allowing indefinite integration over p and T
![]()
Choosing the constant of integration to equal ln p0, where p0 equals one pressure unit, e.g., 1 Torr if pressure is measured in Torr, allows the pressure units to cancel and yields
|
|
This equation is useful for determining DH from a plot of ln (p/p0) vs. 1/T.
If definite integration from p1 to p2 and from T1 to T2 is performed, the result is
|
|
This equation is useful for determining the vapor pressure p2 at temperature T2 given DH and the vapor pressure at one temperature (p1, T1), e.g., the normal boiling point.
In this experiment, (vapor pressure, temperature) data pairs are measured over the pressure range from about 100 Torr to ambient pressure.
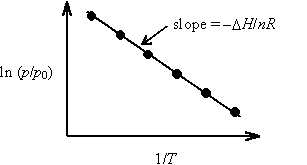
A plot of ln (p/p0) vs. 1/T is constructed, and the slope is interpreted to be according to the indefinite form of the Clausius-Clapeyron equation. The quantity DH/n is interpreted as DvapH, the molar enthalpy of vaporization.
A real Clausius-Clapeyron often shows some slight curvature. This curvature can be analyzed by considering the limitations of the three approximations made during the derivation of Clausius-Clapeyron equation from the rigorously correct Clapeyron equation.
The non-ideal behavior exhibited by a real gas is often represented by the compression factor Z defined by
![]()
For an ideal gas, Z equals unity. For saturated vapor, Z decreases with increasing temperature as the critical point is approached and the gas becomes more like a liquid.
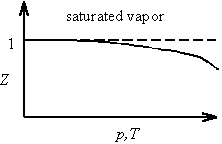
The effect of non-ideal gas behavior on the Clausius-Clapeyron equation can be seen by replacing Videal = nRT/p with Vreal = ZnRT/p. Assuming Z to be independent of p and T for purposes of integration (which is actually a poor numerical approximation, especially near the critical point) yields
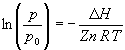
Thus, as temperature increases on a Clausius-Clapeyron plot, Z decreases and the slope (=-DH/ZnR) increases. This results in upward curvature of the plot.
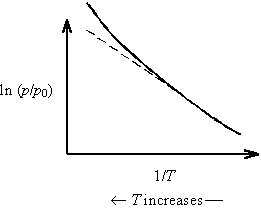
The enthalpy of vaporization can strongly depend on temperature. In fact, DvapH approaches zero as the liquid-vapor co-existence line approaches the critical point, as there is no distinction between liquid and vapor at temperatures and pressures above the critical point!
As temperature increases on a Clausius-Clapeyron plot, DH decreases and the slope (=-DH/ZnR) decreases. This results in downward curvature of the plot.
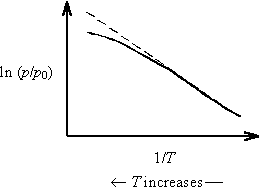
Notice that the non-ideality of real gas and the temperature dependence of DH have opposite effects. It turns out that these effects are each quite significant; however, they very nearly cancel each other out! Hence, Clausius-Clapeyron plots tend to be quite linear. See Waldenstrom et al., Journal of Chemical Education 59, 30 (1982) for a discussion of these effects.
The effect of Vliq can be incorporated into the Clausius-Clapeyron equation by writing
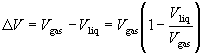
Assuming (1 - Vliq/Vgas) to be independent of temperature for purposes of integration (which is a reasonable approximation only over a small temperature range and far from the critical point) yields
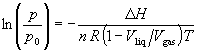
The slope must be multiplied by -(1 - Vliq/Vgas) R to obtain DvapH. Since (1 - Vliq/Vgas) is slightly less than one, neglecting this factor slightly overestimates DvapH, e.g., by less than 0.1%.